In the last few years, we have seen an explosion of cutting-edge medical devices and pharmaceutical interventions come to the market for the treatment of dry eye disease (DED). With all these new treatment options, it can sometimes be confusing to decide what therapies to prescribe. The Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) III report, which was recently released, includes an updated prescribing algorithm based on DED etiology or pathogenic drivers.1 Specifically, there are 10 different DED drivers placed into 3 main categories in the report: (1) tear film deficiencies, (2) eyelid abnormalities, and (3) ocular surface abnormalities.1
Here, I discuss the treatments designated for each of the 3 categories. A caveat: Most DED patients have overlapping causes of DED, so these patients can be in more than 1 of these driver categories at the same time.
Pro Tip: When deciding how to treat DED patients, we want to determine the main cause, based on case history, Ocular Surface Disease Index-6 score, symptoms, clinical signs, and diagnostic findings. Although DED is multifactorial and will almost always require a combination of a variety of different therapies to successfully treat it, I have found that a targeted approach based on its etiology is the best way to start: Tackle the main causes first; then add other treatments as needed.

Category 1: Tear Film Deficiencies
This category has been further divided into lipid, aqueous, and mucin/glycocalyx deficiencies.1 Common therapies here include mainstays, such as artificial tears, lid hygiene, and nutraceuticals.1 Other therapies include ocular surface regenerators, such as amniotic membranes and blood-based therapies, like autologous serum and platelet-rich plasma drops. Also mentioned: tear conservation devices, such as therapeutic scleral contact lenses and punc-tal plugs.1 (It is important to note that punctal plugs are a good option after any inflammatory factors have been addressed.) Tear stimulation, whether from a pharmacologic agent or a device, is a treatment option for all 3 types of tear film deficiencies.
Category 2: Eyelid Anomalies
This category is split into 3 drivers: (1) blinking/lid closure, (2) anterior blepharitis, and (3) meibomian gland dysfunction (MGD). A treatment for patients with blinking/lid closure issues is blinking exercises.1
For anterior blepharitis, treatments include lid hygiene and oral antibiotics.1 If anterior blepharitis is the main cause of DED, I emphasize lid hygiene at home with an in-office lid-cleansing procedure every 3 to 6 months.
A prescription for an antibiotic or antibiotic-ste-roid combination eye drop may also be necessary based on the severity of the clinical presentation. If Demodex is present, lotilaner 0.25% (Xdemvy; Tarsus Pharmaceuticals) can be used.
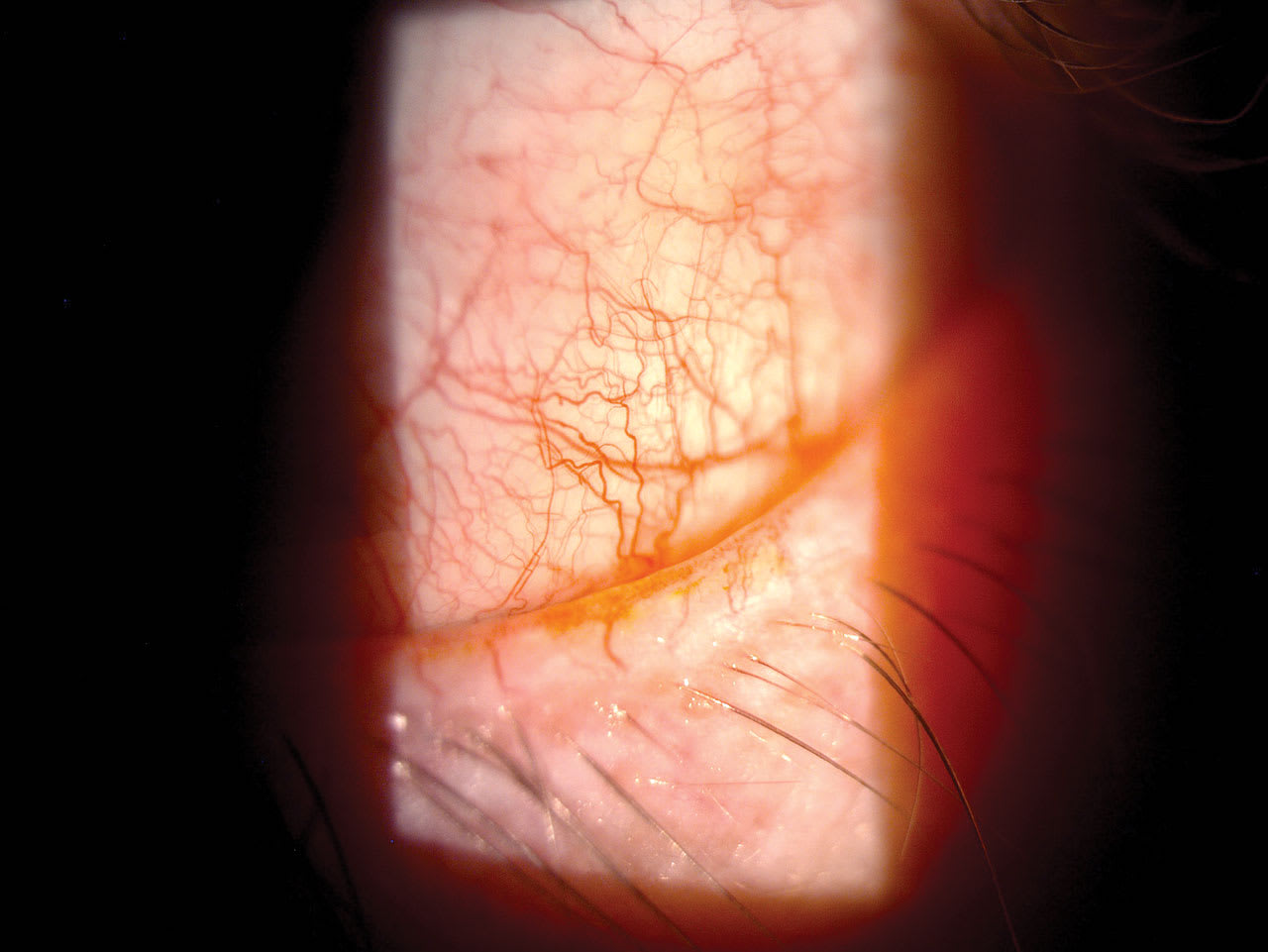
When MGD (the primary cause of evaporative DED) is one of the main drivers, in addition to at-home therapies, the following are the evidence-based therapies: vitamin D3, tear supplementation/stabilization, and in-office tear stimulation devices, when followed by manual expression of the glands.2 Meibomian gland probing could be considered in cases difficult to resolve.3 Other treatments included in the TFOS DEWS III report for MGD are lid margin debridement and selenium sulfide topical preparations.1 (See previous page.)
Telangiectasia was listed as a clinical sign under MGD in the TFOS DEWS III report’s treatment algorithm.1 If ocular rosacea with lid telangiectasia is present, intense pulsed light (IPL) can be a good first-line treatment.4 If IPL is not possible, an oral antibiotic can be prescribed. Remember that ocular rosacea and Demodex blepharitis often occur concurrently because Demodex can be a cause of rosacea.5 Therefore, we should treat the Demodex accordingly as well.
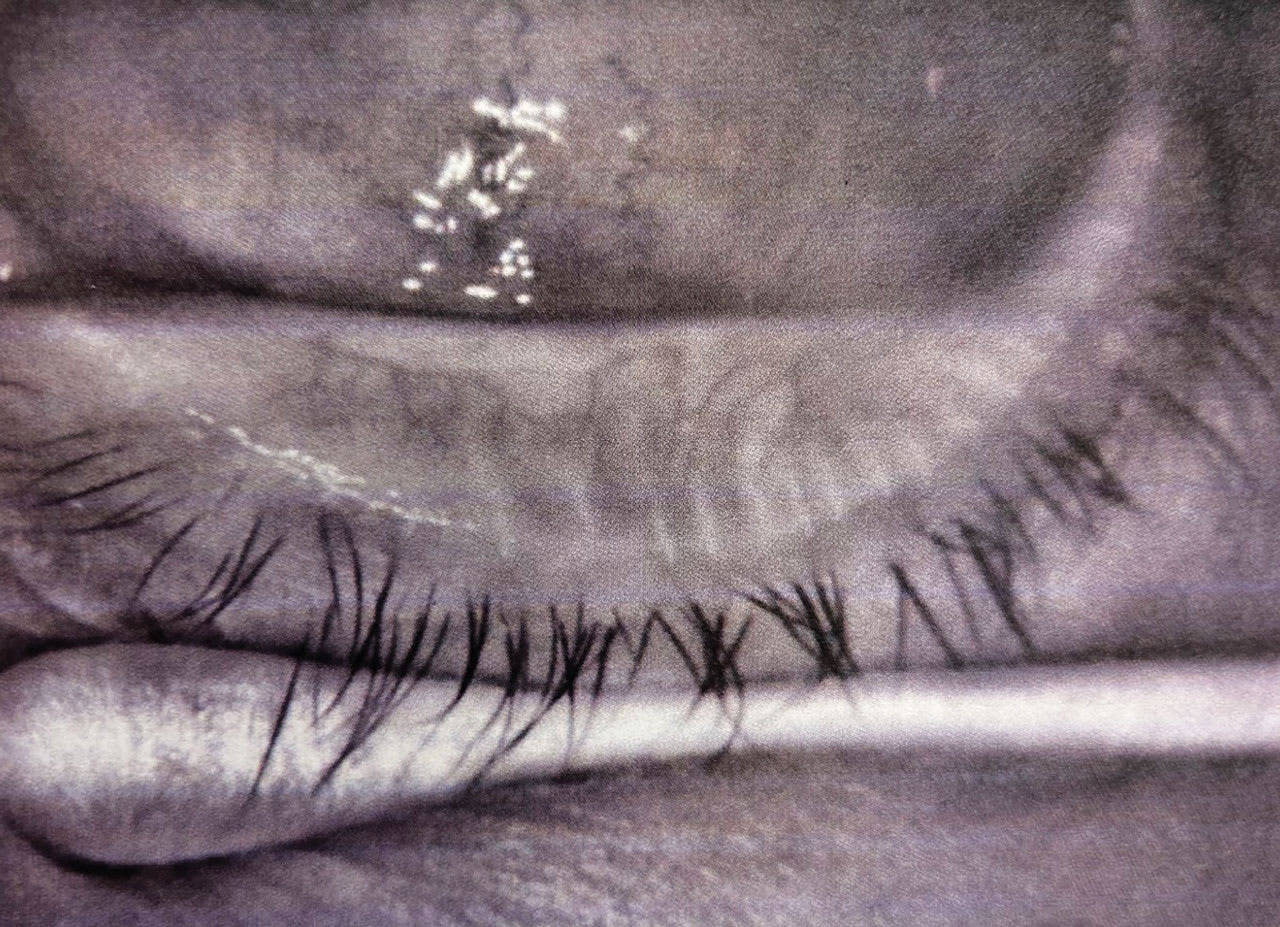
Category 3: Ocular Surface Abnormalities
There are 4 drivers here: (1) anatomic misalignment, (2) neural dysfunction, (3) cellular damage/disruption, and (4) inflammation/oxidative stress.1 Anatomic misalignment refers to conditions such as lagophthalmos, ectropion/entropion, conjunctivochalasis, and pterygium.1 These are all conditions that may require surgical intervention, depending on their severity.
Patients who have inadequate lid closure or lagophthalmos often require overnight therapy. Treatment options here include moisture chamber goggles, ointment, or lid seals/tape.
If a patient has sleep apnea and is using a continuous positive airway pressure (CPAP) machine at night, it is especially important to treat any inadequate lid seal because the continuous air from the CPAP machine can cause further drying of the ocular surface.
An effective way to treat conjunctivochalasis is with high-frequency radiowave electrosurgery.1
Neural dysfunction can include neurotrophic and neuropathic conditions, as well as postsurgical changes. Neurotrophic keratitis (NK) is another consideration when treating ocular surface disease. NK, in its early stage, can look similar to DED.
NK can be definitively diagnosed by performing a corneal sensitivity test. If corneal sensitivity is reduced or absent, the patient has NK. NK can be classified into its own 3 stages, according to the Mackie Classification System. Stage 1 presents as punctate keratitis, Stage 2 is persistent epithelial defects, and Stage 3 presents with a corneal ulcer.
Cellular damage/disruption can be treated with many options. They include vitamin D3, lid hygiene, pharmacological tear stimulation/restoration, and topical anti-inflammatories, among others. If overall inflammation/oxidative stress (including signs of conjunctival hyperemia and other inflammatory markers) is one of the main causative factors, prescription medications, such as immunomodulators and steroids, can be prescribed.

Additional Interventions
In addition to the treatments listed in the
DEWS III Management and Therapy Algorithms, we should also educate patients that lifestyle factors, such as sleep, diet, cosmetic use, contact lens wear, and mental health disorders, can all affect DED. In doing so, we can recommend patients get 7 to 8 hours of sleep per night, eat a Mediterranean diet, remove eye makeup every night, wear daily disposable contact lenses when possible, and seek the help of a mental health specialist if needed.6 OM
References
1. Jones L, Craig JP, Markoulli M, et al. TFOS DEWS III management and therapy report. Am J Ophthalmol. Published online June 2, 2025. doi:10.1016/j.ajo.2025.05.039.
2. Chelnis J, Garcia CN, Hamza H. Multi-frequency RF combined with intense
pulsed light improves signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2023;17:3089-3102. Published 2023 Oct 20. doi:10.2147/OPTH.S426564.
3. Warren NA, Maskin SL. Review of literature on intraductal meibomian gland probing with insights from the inventor and developer: fundamental concepts and misconceptions. Clin Ophthalmol. 2023;17:497-514. doi:10.2147/OPTH.S390085.
4. Shergill M, Khaslavsky S, Avraham S, Kashetsky N, Zaslavsky K, Mukovozov I.
A review of intense pulsed light in the treatment of ocular rosacea. J Cutan Med Surg. 2024;28(4):370-374. doi:10.1177/12034754241254051.
5. National Rosacea Society. Causes of rosacea: Demodex mites & microbes. Accessed June 24, 2025. https://www.rosacea.org/patients/causes-of-rosacea/demodex-mites-and-microbes.
6. Craig JP, Alves M, Wolffsohn JS, et al. TFOS lifestyle report executive summary: a lifestyle epidemic - ocular surface disease. Ocul Surf. 2023;30:240-253. doi:10.1016/j.jtos.2023.08.009.