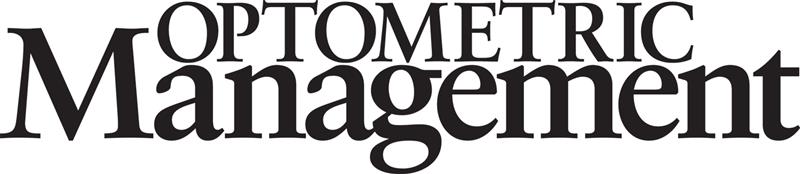This is the second in a series focusing on the diagnosis and treatment of geographic atrophy.
With two novel treatments for geographic atrophy (GA) now available in the United States, it is important to identify patients with GA as well as those most at risk. While treatments require long-term monthly or every-other-month intravitreal injections aimed at slowing the progression of GA, it is also necessary to get a sense of which patients have markers to suggest their GA may expand more rapidly. Optical coherence tomography (OCT) and fundus autofluorescence (FAF) imaging can identify markers not visible on the clinical examination.
Reticular Pseudodrusen
Reticular pseudodrusen (RPD) are a phenotype of drusen that increase GA risk in those with early appearing or intermediate age-related macular degeneration (AMD). In GA patients, the presence of RPD has been associated with faster rates of GA progression.1,2
RPD are small, subtle deposits that can be missed entirely. However, OCT and FAF imaging can readily detect RPD. On OCT, they are small hyperreflective projections that sit on top of the retinal pigment epithelium (RPE) and may project through the photoreceptor integrity line. On FAF, they are small circular deposits in a reticular pattern (see Figure 1).1,2
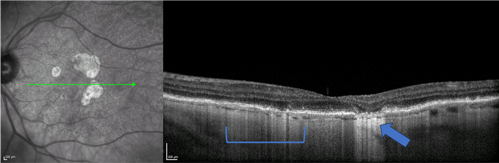
Hyperreflective Columns
On OCT, the RPE is an optical barrier that shields light from easily penetrating the choroid. As the RPE weakens, this optical barrier breaks down, and light columns cascade through the RPE into the choroid. These are called hyperreflective columns. In areas of GA where the RPE has atrophied, there will be well-defined areas of hyperreflectivity in the choroid. Even in areas that are not yet GA, though, hyperreflective columns can be seen as a pre-GA lesion (see Figure 2).3
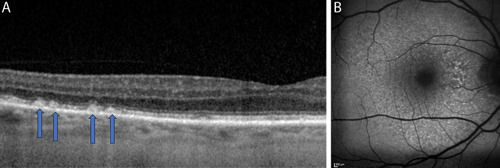
Hyperreflective Foci
Hyperreflective foci are thought to be the OCT equivalent of pigmentary abnormalities and long-known to be a risk for advanced AMD. Hyperreflective foci are pinpoints of hyperreflectivity that often sit on top of large drusenoid pigment epithelial detachments (see Figure 3). They are pigment migrating anteriorly from heavily damaged RPE. Hyperreflective foci significantly increase the risk of GA development.4,5
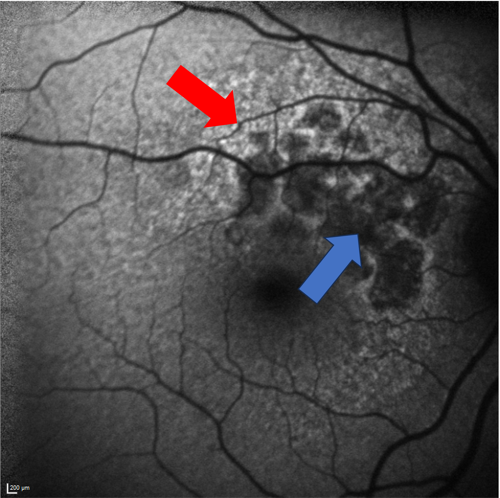
Surrounding Hyper-Autofluorescence
On FAF, GA will appear as well-defined regions of hypo-autofluorescence (dark). This indicates that there is atrophy of the RPE and lack of lipofuscin in the area. If the surrounding RPE is relatively healthy, it will have a uniform gray autofluorescence. If the surrounding RPE already has significant damage, it will accumulate lipofuscin and be hyper-autofluorescence (bright). Therefore, GA that is surrounded by hyper-autofluorescence is more likely to progress quickly, as the surrounding RPE is already damaged (see Figure 4).6
OCT and FAF show much more than meets the eye when it comes to knowing which patients are most likely to experience vision loss from GA. These biomarkers are crucial for patient education, as they determine follow-up times as well as which patients may benefit most from GA treatment.
REFERENCES
- Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–390. doi:10.1016/j.ophtha.2017.08.038
- Agrón E, Domalpally A, Cukras CA, et al. Reticular pseudodrusen status, ARMS2/HTRA genotype, and geographic atrophy enlargement: Age-related eye disease study 2 report 32. Ophthalmology. 2023;130(5):488–500. doi:10.1016/j.ophtha.2022.11.026
- Flores R, Carneiro Â, Tenreiro S, Seabra MC. Retinal progression biomarkers of early and intermediate age-related macular degeneration. Life. 2021;12(1):36. doi:10.3390/life12010036
- Christenbury JG, Folgar FA, O’Connell R V, Chiu SJ, Farsiu S, Toth CA. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038–1045. doi:10.1016/j.ophtha.2012.10.018
- Ouyang Y, Heussen FM, Hariri A, Keane PA, Sadda SR. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120(12):2656–2665. doi:10.1016/j.ophtha.2013.05.029
- Jeong YJ, Hong IH, Chung JK, Kim KL, Kim HK, Park SP. Predictors for the progression of geographic atrophy in patients with age-related macular degeneration: Fundus autofluorescence study with modified fundus camera. Eye (Lond). 2014;28(2):209–218. doi:10.1038/eye.2013.275
This editorially independent content is sponsored by